When a patient walks into your office asking why their prescription now looks different, or complains their blood pressure isn’t as controlled since switching meds, you’ve likely faced the real-world tension between cost savings and clinical confidence. Generic medications are now the backbone of U.S. prescribing-making up 90% of all prescriptions-but provider experiences with them aren’t as simple as ‘same drug, cheaper price.’
What Providers Actually See in Practice
Most primary care doctors, cardiologists, and endocrinologists report smooth transitions when switching patients from brand-name statins like Lipitor to atorvastatin generics. The clinical outcomes? Nearly identical. A 2019 JAMA Internal Medicine study tracking over 10 drugs found no meaningful difference in heart attacks, hospitalizations, or medication discontinuation between brand and generic versions. For common conditions like high cholesterol, hypertension, or type 2 diabetes, generics work just as well-and patients are more likely to stick with them because they cost less. But that’s not the whole story. Providers treating patients on narrow therapeutic index (NTI) drugs see a different reality. These are medications where even tiny changes in blood levels can cause serious harm. Think warfarin, levothyroxine, cyclosporine, and certain antiepileptic drugs like lamotrigine. For these, many clinicians avoid automatic substitution. One neurologist in Ohio reported three patients with previously stable epilepsy had breakthrough seizures after switching from brand Lamictal to a generic version. All three returned to seizure control only after switching back. That’s not theory-it’s clinical reality.The Orange Book Isn’t Always Enough
The FDA’s Orange Book lists therapeutic equivalence ratings: ‘AB’ means the generic is considered interchangeable with the brand. But not all generics are created equal. In 2016, the FDA downgraded two generic versions of Concerta (a brand-name ADHD medication) from AB to BX after receiving dozens of patient complaints about reduced effectiveness. The agency reviewed adverse event reports, ran lab tests, and consulted experts before making the change. That’s rare-and telling. It shows that bioequivalence studies (which measure how fast and how much drug enters the bloodstream) don’t always capture what patients feel. One pill might meet the 80-125% bioequivalence range, but if the inactive ingredients change how the drug is absorbed over time, or if the tablet breaks down inconsistently, patients notice. Providers in mental health and neurology say they’ve seen this with SSRIs too-patients report mood swings or increased anxiety after switching generics, even when labs show normal blood levels.State Laws Make It a Patchwork
You can’t talk about generic substitution without talking about state laws. In 19 states, pharmacists can substitute generics automatically without telling the patient. In seven states and Washington D.C., they’re required to get patient consent first. And in 24 states, pharmacists face no legal protection if a patient has a bad reaction after a substitution. This inconsistency creates confusion. A patient in New York gets a generic substitution without being told. A week later, they move to Texas and refill their prescription-only to get a different generic from a different manufacturer. Suddenly, they’re on three different versions of the same drug in six months. Providers hear: “I took the same medicine, but it didn’t work this time.”Authorized Generics: The Hidden Middle Ground
Many providers don’t realize that authorized generics exist. These are the exact same drug as the brand-name version, made by the original manufacturer but sold under a generic label. For example, the maker of Lipitor might sell atorvastatin under its own name, without the brand logo. These are often preferred by clinicians because they’re identical in formulation, packaging, and manufacturing to the brand. A 2020 Johns Hopkins study found that patients using authorized generics had nearly the same outcomes as those on the brand-name drug-far closer than those on regular generics. But because authorized generics are often priced higher than standard generics, insurers don’t always push them. Providers who know about them often ask pharmacists specifically for them, especially for NTI drugs.Patients Believe What They Feel
Here’s the gap: patients trust their bodies more than their prescriptions. A 2024 Greek survey found that 68% of women followed their doctor’s advice to switch to generics-but only 64% of men did. Why? Because men were more likely to report side effects after switching, even when clinical data showed no difference. And patients who’ve had a bad experience with one generic? They’ll refuse all generics after that. One nurse practitioner in Minnesota told me about a 68-year-old man who refused to take any generic blood pressure pills after his first one made him dizzy. He’d been on the brand for years, switched to a generic, felt awful, and went back to the brand. His doctor explained it was likely a coincidence-but the patient didn’t care. He’d felt it. That’s not irrational. It’s human.What Works: Education, Not Mandates
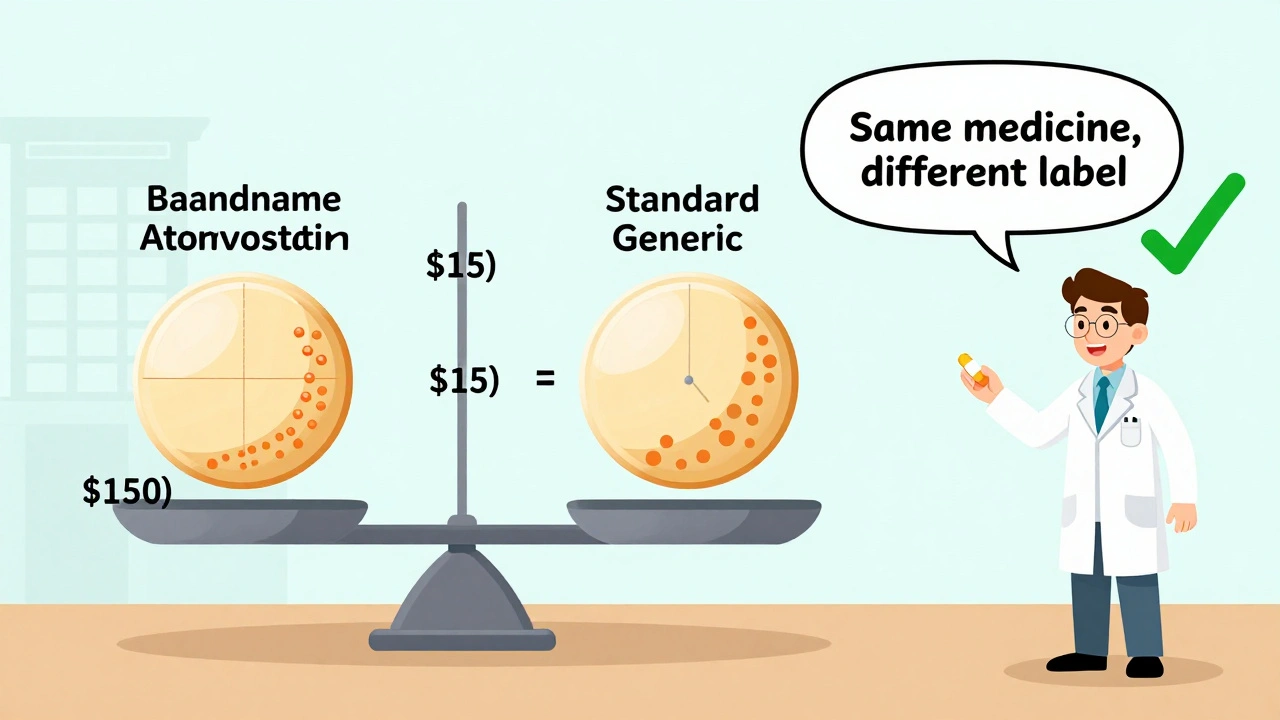
The best providers don’t force substitutions. They explain them. A simple five-minute conversation before switching can increase patient acceptance by over 50%. Saying something like, “This generic has the same active ingredient as your old pill. It’s been tested to work the same way. The only difference is it costs $15 instead of $150,” changes the whole dynamic. Providers who include patients in the decision-asking, “Do you want to try this cheaper version?”-see higher adherence and fewer complaints. One clinic in Ohio tracked this: after implementing a standardized counseling script, generic switch success rates jumped from 62% to 89% in three months. Pharmacists are critical here too. When a patient gets a new generic version, the pharmacist can say, “This is the same medicine, just made by a different company. The pill looks different, but it works the same.” That simple reassurance prevents panic.Where Providers Should Draw the Line
For most medications-statins, ACE inhibitors, metformin, SSRIs-generics are safe and effective. No need to overthink it. But for these, proceed with caution:- Antiepileptic drugs (lamotrigine, phenytoin, carbamazepine)
- Immunosuppressants (cyclosporine, tacrolimus)
- Thyroid hormone (levothyroxine)
- Anticoagulants (warfarin)
- Some psychiatric medications (especially if patient has had prior issues)
The Bigger Picture: Cost, Access, and Equity
Generics aren’t just about saving money for insurers. They’re about saving lives for patients who can’t afford brand-name drugs. A 2001-2003 study found patients who started on generics were 13% more likely to keep taking their meds than those on brand-name versions. That’s not a small difference-it’s the difference between controlled diabetes and hospitalization. Medicare Part D now pushes generics hard. Over 90% of prescriptions for seniors are generic. And with the Inflation Reduction Act, that number is expected to rise. But if providers don’t manage the transition well, patients will stop taking their meds-not because they’re ineffective, but because they’re confused, scared, or feel unheard.What’s Next?
The FDA is now using real-world data from its Sentinel Initiative to track outcomes after generic switches-not just bioequivalence numbers. Machine learning models are being trained to predict which patients are most likely to have trouble switching based on age, comorbidities, and prior medication history. Meanwhile, supply chain issues from the pandemic exposed how fragile generic drug production is. Over 80% of active ingredients come from overseas. When factories in India or China shut down, shortages follow. That’s why some hospitals now keep a small stock of authorized generics as a backup. The future isn’t about choosing brand over generic. It’s about choosing the right generic, at the right time, with the right conversation.Are generic medications really as effective as brand-name drugs?
For most medications, yes. The FDA requires generics to deliver the same amount of active ingredient into the bloodstream at the same rate as the brand. Studies tracking outcomes for drugs like statins, blood pressure meds, and antidepressants show no meaningful difference in effectiveness or safety. But for narrow therapeutic index drugs-like warfarin, levothyroxine, or antiepileptics-small variations can matter. Providers often avoid automatic substitution for these.
Why do some patients say generics don’t work for them?
Patients sometimes report differences due to changes in inactive ingredients, pill size, or how the drug is absorbed over time. Psychological factors also play a role-if someone believes generics are inferior, they may interpret normal side effects as the drug not working. In rare cases, especially with complex formulations like extended-release pills, bioequivalence studies may miss subtle differences. That’s why providers monitor patients closely after a switch.
Can pharmacists substitute generics without telling me?
It depends on your state. In 19 states, pharmacists can switch to a generic without notifying you. In seven states and D.C., they must get your consent first. Always check your prescription label-if the manufacturer or pill appearance changes unexpectedly, ask your pharmacist. You have the right to know what you’re taking.
What’s the difference between a generic and an authorized generic?
An authorized generic is made by the same company that produces the brand-name drug, just sold without the brand name. It’s identical in every way-same ingredients, same factory, same packaging. A regular generic is made by a different company and may have different inactive ingredients or manufacturing processes. Authorized generics are often preferred for sensitive medications because they eliminate uncertainty.
Should I ask my doctor to write “do not substitute” on my prescription?
If you’re taking a drug with a narrow therapeutic index-like warfarin, levothyroxine, or an antiepileptic-it’s reasonable to ask. Many providers do this routinely for these medications. For common drugs like statins or metformin, it’s usually unnecessary. Talk to your doctor about your concerns. They can help you decide what’s right for your situation.
Do generics cause more side effects?
Large studies show no significant increase in side effects with generics compared to brand-name drugs. However, some patients report new or different side effects after switching-often because the inactive ingredients (like fillers or dyes) changed. These aren’t usually dangerous, but they can be uncomfortable. If you notice new symptoms after a switch, tell your provider. It doesn’t mean the drug isn’t working-it just means your body might be reacting to something new.
Why do generics look different every time I refill?
There are often multiple generic manufacturers for the same drug. Each one makes their own version, so pills may differ in color, shape, or size. This doesn’t affect effectiveness, but it can confuse patients. Always check the label for the drug name and dosage. If you’re unsure, ask your pharmacist to confirm it’s the same medication.





10 Comments