For decades, leukemia and lymphoma were treated with one main tool: chemotherapy. It was brutal, unpredictable, and often left patients weaker than before. But in 2025, that’s no longer the story. A quiet revolution has taken over blood cancer care-targeted therapy and CAR T-cell therapy are now changing survival rates, reducing side effects, and giving people back years they thought they’d lost.
How Targeted Therapies Work: Hitting the Right Switch
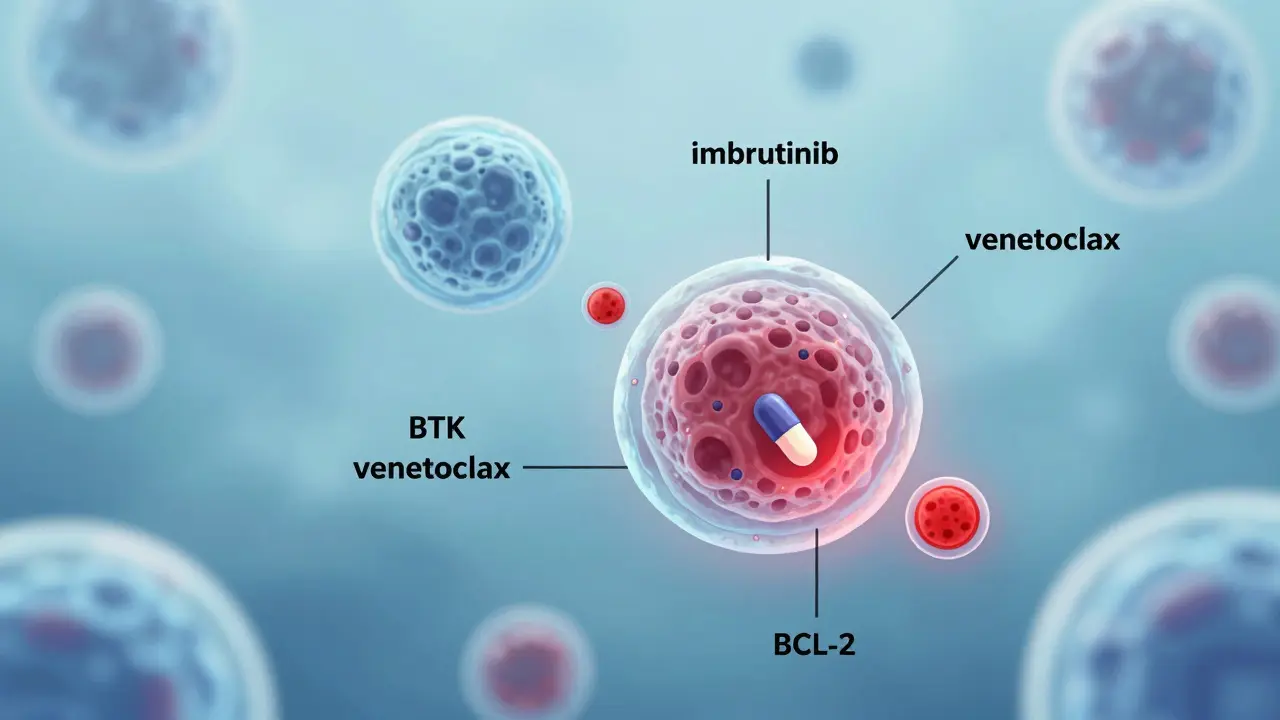
Targeted therapies don’t blast every fast-growing cell in your body. They’re like precision tools-designed to block just the signals that cancer cells use to survive. In chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL), the cancer depends heavily on a protein called BTK. Drugs like ibrutinib and acalabrutinib shut down BTK, starving the cancer of the signals it needs to grow. Another key player is BCL-2, a protein that keeps cancer cells from dying. Venetoclax blocks BCL-2, forcing those cells to self-destruct.
These drugs are taken orally, usually once a day. No IV lines. No hospital stays. For many patients, this means living with cancer rather than being crushed by treatment. In clinical trials, combinations like venetoclax + ibrutinib have led to deep remissions in over 80% of untreated CLL patients. That’s not just better survival-it’s a return to normal life.
But it’s not perfect. Resistance builds over time. After 3 to 5 years, many patients see their cancer start to grow again. Those with a specific genetic flaw-del(17p) or TP53 mutation-are more likely to develop resistance early. That’s why doctors now use these drugs in combinations and time-limited courses, not forever.
CAR T-Cell Therapy: Rewiring Your Immune System
If targeted therapy is a scalpel, CAR T-cell therapy is a full-scale rebuild. It starts with a simple blood draw. Your T cells-the immune system’s frontline fighters-are collected. In a lab, scientists give them a new GPS: a chimeric antigen receptor (CAR) that lets them recognize CD19, a protein found on almost all B-cell lymphomas and leukemias.
These modified T cells are multiplied-sometimes into hundreds of millions-then infused back into your body. Once inside, they hunt down cancer cells like living missiles. Unlike chemo, they can stick around for years, keeping watch. This isn’t just treatment. For some, it’s a cure.
Yescarta (axicabtagene ciloleucel) and Kymriah (tisagenlecleucel) were the first two approved in 2017. Now, newer versions like KITE-363 and KITE-753 target two antigens at once-CD19 and CD20. This dual approach reduces the chance the cancer escapes by losing one target. In a recent trial for mantle cell lymphoma, a fixed dose of LV20.19 CAR T-cells led to a 100% response rate and 88% complete remission. That’s unheard of in relapsed disease.
Who Benefits Most? The Real-World Picture
Targeted therapies work best for patients with CLL, SLL, and some types of follicular lymphoma. They’re now first-line for many, replacing chemoimmunotherapy. For aggressive lymphomas like diffuse large B-cell lymphoma (DLBCL), CAR T-cell therapy is reserved for when other treatments fail-but that’s changing fast.
According to ZUMA-7 data presented at ASH 2025, using Yescarta as a second-line treatment (after one prior therapy) gave patients a 42.6% chance of surviving four years. That’s nearly double what chemotherapy offered just five years ago.
But not everyone qualifies. CAR T-cell therapy requires a patient to be well enough to wait 3 to 5 weeks for manufacturing. It’s not for those with severe heart or lung disease. And it’s not cheap. The average cost is $373,000 to $475,000 per treatment. Even with insurance, out-of-pocket costs can hit $15,000 to $25,000 a month for targeted drugs over time.
One real-world problem: access. While 89% of NCI-designated cancer centers offer CAR T-cell therapy, only 32% of community clinics do. Many patients travel hundreds of miles-or delay treatment-because their local hospital can’t handle the complexity.
Toxicity: The Hidden Cost of Breakthroughs
These therapies are powerful-but dangerous if not managed right.
CAR T-cell therapy can trigger cytokine release syndrome (CRS), a massive immune overreaction. Symptoms include high fever, low blood pressure, and trouble breathing. About 20-40% of patients experience neurotoxicity-confusion, seizures, or trouble speaking. These usually resolve with treatment, but they require ICU-level care.
Venetoclax carries its own risk: tumor lysis syndrome. When cancer cells die too fast, they flood the bloodstream with waste products. That can crash kidney function. That’s why patients start on low doses and are often hospitalized during the first few weeks.
Doctors now use strict grading systems and 24/7 hotlines from drug manufacturers to manage these risks. Kite’s support program reports 95% satisfaction among patients and families navigating this process.
The Future: Earlier Use, Better Safety, Lower Cost
The next five years will change everything. Researchers are testing CAR T-cell therapy as a first-line treatment for high-risk lymphomas. A 2025 ASCO survey found 68% of hematologists believe this will be standard by 2030.
Newer CAR T designs aim to reduce toxicity. Bicistronic therapies like KITE-363 use two co-stimulatory domains (CD28 and 4-1BB) to make the cells more stable and less likely to overactivate. Early data suggests these could be given outside the hospital-turning a 3-week ICU stay into an outpatient procedure.
Manufacturing is also speeding up. Some labs now produce CAR T-cells in under 10 days, not 3 weeks. That opens the door for more patients, including those who are sicker or older.
Cost is the biggest barrier. But with more competitors entering the market-like AstraZeneca’s acalabrutinib and AbbVie’s venetoclax-prices are starting to drop. Generic versions of BTK inhibitors are expected by 2027. And payers are starting to fund outcomes-based contracts: you pay less if the treatment doesn’t work.

What This Means for Patients Today
If you or someone you know has leukemia or lymphoma, here’s what you need to know in 2025:
- Targeted therapies are now standard for CLL/SLL. Ask about venetoclax + ibrutinib or venetoclax + obinutuzumab.
- If you’ve had two or more treatments fail, CAR T-cell therapy is likely your best shot at long-term remission.
- Don’t wait to ask about genetic testing. Mutations like TP53 or del(17p) change which treatment works best.
- Find a center with experience. CAR T-cell therapy requires specialized teams. Ask if they’ve treated at least 20 cases in the last year.
- Ask about financial help. Most manufacturers offer copay assistance and patient navigation services.
These therapies aren’t magic. They don’t work for everyone. But for the first time in history, we’re not just managing blood cancers-we’re changing their course. Some patients are living 10, 15, even 20 years longer than doctors expected. That’s not a future dream. It’s happening right now.
What’s the difference between targeted therapy and CAR T-cell therapy?
Targeted therapy uses pills or infusions to block specific proteins cancer cells need to survive, like BTK or BCL-2. It’s a continuous treatment that slows or stops cancer growth. CAR T-cell therapy is a one-time treatment where your own immune cells are genetically changed to hunt and kill cancer cells. It’s not a drug-it’s a living medicine that can last for years.
Are these therapies available everywhere?
Targeted therapies are widely available through oncologists and community clinics. CAR T-cell therapy is only offered at certified centers with ICU capabilities and specialized teams. In the U.S., about 89% of major cancer centers offer it, but only 32% of community hospitals do. Patients often travel to academic medical centers for treatment.
How long does CAR T-cell therapy take from start to finish?
The process takes about 6 to 8 weeks total. First, your T cells are collected (a few hours). Then, they’re sent to a lab for genetic modification and growth-this takes 3 to 5 weeks. After that, you receive conditioning chemo for 2-3 days, then the CAR T infusion. You stay in the hospital for 1-2 weeks for monitoring. Recovery at home can take 2-6 months.
Do these therapies cure cancer?
For some patients, yes. In relapsed or refractory lymphoma, CAR T-cell therapy has led to durable remissions lasting over 10 years in a small but significant group. Targeted therapies rarely cure but can control cancer for many years. For CLL, combinations like venetoclax + ibrutinib can lead to deep remissions where cancer is undetectable-even without ongoing treatment.
What are the biggest risks with CAR T-cell therapy?
The two biggest risks are cytokine release syndrome (CRS) and neurotoxicity. CRS causes high fever, low blood pressure, and breathing issues. Neurotoxicity can lead to confusion, seizures, or trouble speaking. Both are serious but usually reversible with prompt treatment. About 1 in 5 patients need ICU care. Long-term immune suppression is also common, requiring regular IVIG infusions to prevent infections.
Why are these therapies so expensive?
CAR T-cell therapy is expensive because it’s custom-made for each patient. It involves complex lab work, specialized staff, and months of development. The manufacturing process can’t be scaled like a pill. Targeted drugs are costly due to patent protection and high R&D costs. But prices are starting to fall as more companies enter the market and generic versions emerge.
What Comes Next?
The next frontier isn’t just better drugs-it’s smarter timing. Doctors are starting to use CAR T-cell therapy earlier, even before patients relapse. Trials are testing CAR T in first-line treatment for high-risk lymphoma. If successful, it could replace chemotherapy entirely for some.
Another big shift: combining therapies. Some patients get a BTK inhibitor first, then CAR T if they relapse. Others are being tested with CAR T plus checkpoint inhibitors to boost response. The goal isn’t just to kill cancer-it’s to train the immune system to remember it forever.
For patients, this means hope. For families, it means more time. For doctors, it means a new responsibility: choosing the right tool for the right person at the right time. We’re not just treating cancer anymore. We’re rewriting its story.



8 Comments