Authorized generics let brand-name drug makers launch their own cheaper versions during the first generic's exclusivity period, undercutting competition and keeping prices high. Here's how it works-and why it hurts patients.
Tag: Hatch-Waxman Act
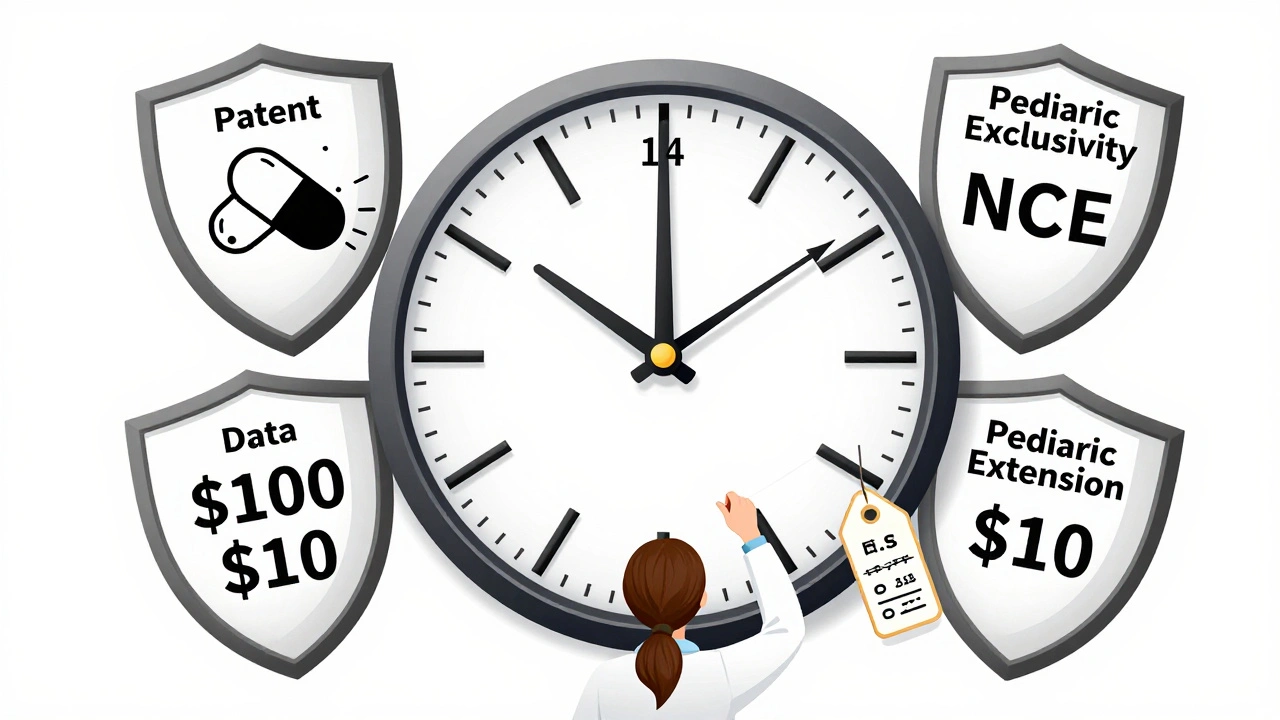
Generic drug exclusivity periods vary widely by country, affecting when affordable versions hit the market. The U.S. offers complex protections including 180-day exclusivity for first challengers, while the EU uses a fixed 8+2+1 model. These rules shape global drug prices and access.
Brand manufacturers produce their own generic versions to retain market share after patents expire. These authorized generics are identical to the original drug but sold under a different label, often at a slightly lower price. Learn how they work, why they're controversial, and what it means for your prescription costs.
Litigation in Generic Pharmaceutical Markets: How Patent Disputes Delay Affordable Medicines
Nov, 12 2025Patent litigation in the generic drug market is delaying affordable medicines for years. Learn how Hatch-Waxman, Orange Book listings, and pay-for-delay settlements are reshaping access to life-saving drugs.