When a popular brand-name drug loses its patent, prices usually crash. Patients expect to pay 80% less. But here’s the twist: the same company that made the brand-name pill often launches its own generic version - same pill, same factory, same ingredients, just a different label. This isn’t a loophole. It’s a legal, well-documented strategy called authorized generics.
What Exactly Is an Authorized Generic?
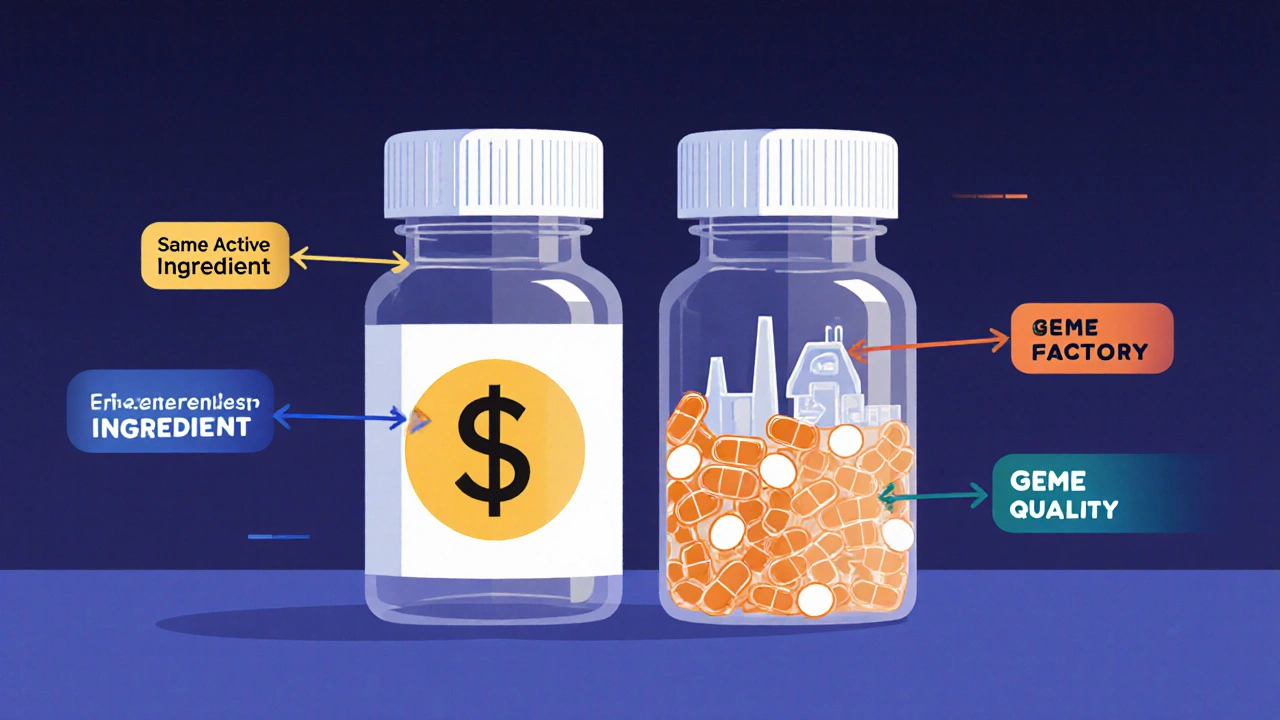
An authorized generic is the exact same medication as the brand-name drug - same active ingredient, same inactive ingredients, same size, shape, color, and how it works in your body. The only differences? The packaging and the name on the box. No clinical trials are needed because the manufacturer already proved the drug’s safety and effectiveness years ago. The FDA treats it as a generic, but it comes from the original maker.
This isn’t a copycat product made by a competitor. It’s the real thing, straight off the same production line. Pfizer, Johnson & Johnson, and AbbVie have all done this. For example, when Eli Lilly launched an authorized generic of Cialis (tadalafil) in 2018, it was identical to the branded version - just sold under a different name at a slightly lower price.
Why Do Brand Companies Do This?
It’s not charity. It’s survival.
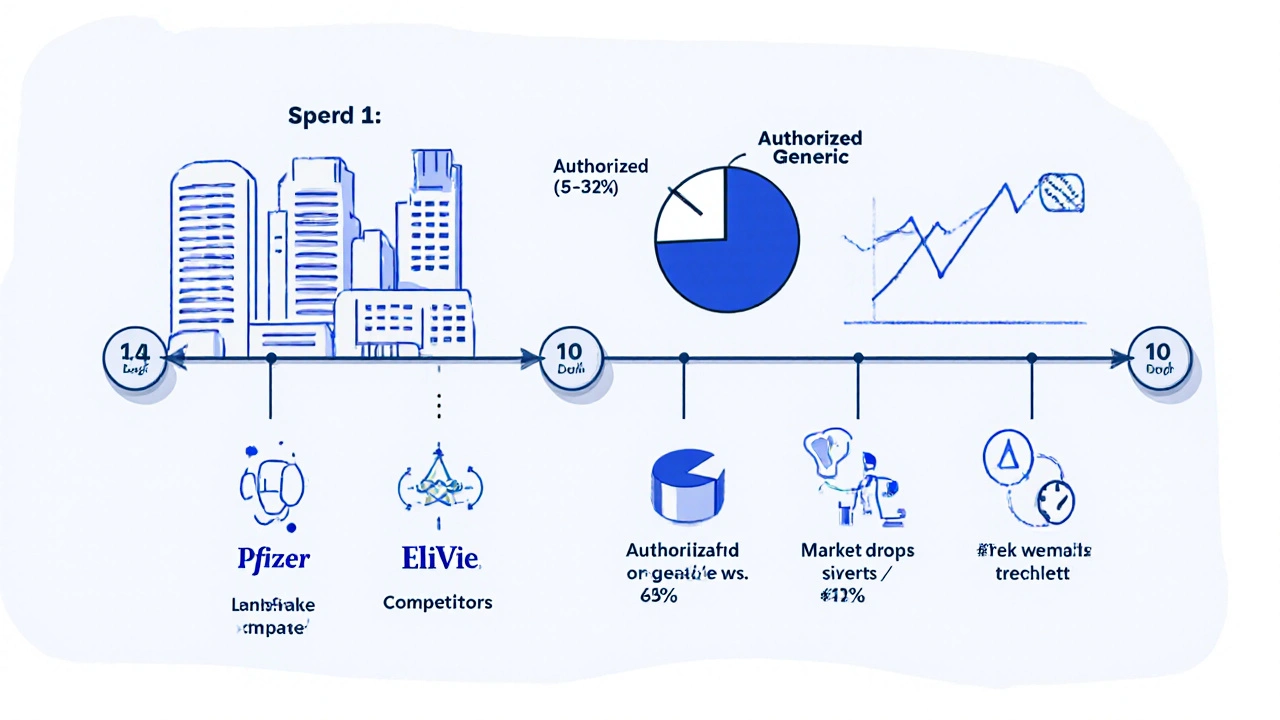
Once a patent expires, multiple generic companies can rush in. Prices can drop 80-85% in the first year. That’s a massive revenue hit. If the brand company does nothing, it loses nearly all its sales. But if it launches its own generic, it keeps a chunk of the market - often 15-35% in the first year. That’s millions, sometimes billions, in retained revenue.
They also control quality. Traditional generics are made by other companies, sometimes overseas, with different standards. An authorized generic? Same facility, same workers, same quality checks. Patients who trust the brand can still get the same product - just cheaper.
And here’s the strategic edge: authorized generics skip the 180-day exclusivity period that the first generic company gets under the Hatch-Waxman Act. That means the brand manufacturer can launch on day one - right alongside competitors. Teva did this with Copaxone in 2019 and captured 22% of the generic market in just three months.
How Is It Made? The Process
The process starts years before the patent expires.
Companies begin planning 24 to 36 months ahead. They don’t need to build new factories. They don’t need to reformulate. All they do is prep their existing production line for a second label. That means changing the box, the blister pack, the instructions - but nothing about the pill itself.
They file an Abbreviated New Drug Application (ANDA) with the FDA, using their own existing data. This cuts the approval time from 17 months (typical for a new generic applicant) down to 6-9 months. The FDA already knows the drug inside and out. Why make them retest it?
Costs? Around $15-25 million to set up the regulatory, labeling, and marketing infrastructure. But the return comes fast - usually within 14 months. That’s because they’re not starting from zero. They’re protecting what they already own.
How It Affects You - The Patient
On paper, authorized generics sound like a win. You get the same drug, same quality, at a lower price. But in practice, it’s more complicated.
Here’s the catch: they’re not always the cheapest option. An authorized generic might cost $85, while a traditional generic from another company is $30. That’s a $55 difference. Many patients don’t realize they’re the same pill. They see the brand name on the box and think, “This must be better.”
A 2023 Kaiser Family Foundation survey found that 71% of patients preferred authorized generics - but 64% didn’t know they were made by the same company as the brand. That’s confusion. Independent pharmacists report patients asking, “Why is this generic so expensive?”
And the price drop? It’s not as deep as it could be. Harvard’s Dr. Aaron Kesselheim found that when authorized generics enter the market, prices fall only 32% on average. Without them, prices drop 68%. That’s because the brand manufacturer is still in the game - they’re not letting the market fully open up.
Controversy and Regulation
This strategy isn’t without criticism.
The Federal Trade Commission (FTC) has sued companies for using authorized generics to block competition. In 2017, Actavis settled for $448 million after the FTC accused it of launching an authorized generic of Namenda to scare off rivals. The FTC argued this wasn’t competition - it was market manipulation.
PhRMA, the brand drug industry group, defends it as “increasing access.” They point to FDA data showing 99.7% bioequivalence between brand and authorized generics. But critics say it’s a shell game - the same company owns both, so they control the price floor.
The law allows it. The FDA allows it. But the spirit of the Hatch-Waxman Act - which was meant to lower prices through competition - is being tested.
What’s Changing Now?
Authorized generics are growing fast. Between 2020 and 2023, the top five drug companies launched 47 of them. That’s up 28% year over year.
And now they’re moving into more complex drugs. In 2023, Johnson & Johnson launched the first authorized generic of a long-acting injectable - Invega Sustenna. These aren’t simple pills anymore. They’re specialized treatments that require advanced manufacturing. Traditional generic companies often can’t replicate them. That’s where the brand manufacturer has a huge advantage.
Even biologics - complex, protein-based drugs - are next. Amgen launched the first authorized biosimilar (a version of its own Enbrel) in 2023. This could become the new norm.
By 2027, analysts predict authorized generics will make up 25-30% of the entire generic drug market - up from 18% in 2022. That’s not a blip. It’s a structural shift.
What Should You Do?
If you’re on a brand-name drug that’s about to go generic, ask your pharmacist:
- Is there an authorized generic available?
- Is there a cheaper traditional generic?
- Are they the same thing?
Don’t assume the “generic” label means cheaper. Sometimes it’s just a rebranded version of what you’ve always taken. And sometimes, the real bargain is the one made by a different company.
Know your options. Ask questions. Your wallet - and your health - will thank you.
Are authorized generics the same as the brand-name drug?
Yes. Authorized generics are identical to the brand-name drug in active ingredients, dosage, strength, shape, size, and how they work in your body. The only differences are the packaging and the name on the label. They’re made in the same factory, by the same company, using the same process.
Why is an authorized generic sometimes more expensive than a regular generic?
Because the brand manufacturer still controls the price. They set the authorized generic’s price just below the brand version - often $5-10 cheaper - but still higher than competing generics made by other companies. This lets them keep revenue while appearing to offer savings. The cheapest option is usually the traditional generic from a different manufacturer.
Do authorized generics delay cheaper generics from entering the market?
They don’t legally block them, but they can reduce their impact. By launching on day one of patent expiry, the brand manufacturer captures market share before other generics gain traction. This lowers overall price competition. Studies show markets with authorized generics see only 32% price drops, compared to 68% when only traditional generics compete.
Is it safe to switch from a brand-name drug to its authorized generic?
Yes. Authorized generics are FDA-approved and bioequivalent to the brand. The FDA requires them to perform the same way in your body. Many patients report no difference at all - and some prefer them because they’re familiar. Always check with your doctor or pharmacist if you have concerns.
Why don’t more companies use authorized generics?
They do - and it’s growing. About 68% of top-selling brand drugs that lost patents between 2018 and 2022 had authorized generic versions launched by the original maker. It’s a smart business move for companies with strong manufacturing, regulatory teams, and large patient bases. Smaller companies often don’t have the resources to manage it.
Can I ask my pharmacy to give me the authorized generic instead of the brand?
Yes. Ask your pharmacist if an authorized generic is available for your prescription. They can check the label and tell you if it’s made by the brand company. If you’re paying a high copay, you might be able to switch to a cheaper traditional generic instead. Always compare prices - don’t assume the “generic” label means the lowest cost.




9 Comments