When a brand-name drug’s patent expires, the promise of cheaper generics kicks in. But what if the brand company itself launches a generic version-same pills, same factory, same price-just with a different label? That’s an authorized generic. And it’s reshaping how competition works in the pharmaceutical industry, often in ways that hurt the very system Congress designed to lower drug prices.
What Exactly Is an Authorized Generic?
An authorized generic is the exact same drug as the brand-name product, made by the same company, in the same facility, and sold under a generic name. It doesn’t need new FDA approval because it’s not a new drug-it’s just repackaged. The brand company either sells it directly or licenses it to a third party. The catch? It hits the market at the same time as, or even before, the first independent generic manufacturer. This isn’t some loophole. It’s legal under the Hatch-Waxman Act of 1984, which was meant to speed up generic access. The law gives the first generic company that challenges a patent a 180-day exclusivity period. During that time, they’re supposed to be the only generic on the market, letting them capture most of the sales and recoup their legal costs. But authorized generics break that promise.How Authorized Generics Kill the Incentive to Challenge Patents
Generic companies spend millions suing brand-name firms over patents. They risk losing everything-time, money, legal fees-just to get that 180-day window. If they win, they get to be the only generic for half a year. That’s the reward. That’s the incentive. But when the brand company drops its own authorized generic into the market during that window, everything changes. Instead of capturing 80-90% of the generic market, the first-filer generic now shares it with a product that’s chemically identical but priced higher than a true generic. The FTC found that authorized generics grab 25-35% of sales during the exclusivity period. That means the real generic company’s revenue drops by 40-52%. That’s not just a hit-it’s a dealbreaker. For smaller generic manufacturers, that lost revenue can mean the difference between staying in business or shutting down. Teva reported a $275 million revenue shortfall in 2018 from just one authorized generic. When the math doesn’t add up, companies stop filing patent challenges. And that’s exactly what brand-name companies want.The Silent Deal: Reverse Payments and Authorized Generics
Here’s where it gets shady. Between 2004 and 2010, about 25% of patent settlements between brand and generic companies included an agreement: the brand won’t launch its own generic, and the generic won’t enter the market for months-or even years. These are called "reverse payment" deals. The brand pays the generic to stay out, and the consumer pays more. The FTC called these "the most egregious form of anti-competitive behavior." In one case, a settlement delayed generic entry by over three years. The drug stayed at brand price, while the generic sat on the sidelines. The brand didn’t even need to launch an authorized generic-they just promised not to. That’s collusion dressed up as a business deal. Even when there’s no explicit payment, the threat of an authorized generic is enough to scare off challengers. A 2023 study showed that generic companies are far less likely to sue if they know the brand might drop an authorized version. Why risk $50 million in legal fees if you’ll only make $10 million after the brand steals half your sales?
Who Benefits? (Spoiler: Not Patients)
PhRMA, the brand-name drug lobby, claims authorized generics help consumers by adding "more competition." But that’s misleading. Authorized generics don’t slash prices like true generics do. They’re priced 15-20% below the brand but 25-30% above the real generic. That creates a pricing sandwich: brand at the top, authorized generic in the middle, true generic at the bottom. Patients and insurers end up paying more than they should. Pharmacy benefit managers (PBMs) like them because they offer a middle-tier option. But that’s not about saving money-it’s about managing formularies. For patients, it’s a trap. If your insurance only covers the authorized generic, you might think you’re getting a cheap option. But you’re not. You’re getting the brand’s product at a slightly lower price, while the real generic-cheaper and just as safe-is sidelined. The Congressional Budget Office estimated that banning authorized generics during the 180-day window would increase generic challenges by 5-7%. That could save Medicare $4.7 billion over ten years. Yet the practice continues.Regulators Are Watching-But Not Stopping It
The FTC has been sounding the alarm since 2011. Their reports show clear evidence: authorized generics reduce generic competition, suppress innovation, and inflate drug prices. In 2022, the FTC declared that "agreements to delay authorized generic entry" are a top enforcement priority. Since 2020, they’ve opened 17 investigations into these arrangements. The Supreme Court took a step in the right direction in 2013 with FTC v. Actavis, ruling that reverse payments can violate antitrust law. But they didn’t specifically ban authorized generics. That left the door open. Congress has tried to fix it. The Preserve Access to Affordable Generics Act has been reintroduced multiple times, most recently in March 2023. It would make it illegal to strike deals that delay authorized generic entry. But it keeps getting stalled. Meanwhile, authorized generic launches have dropped-from 42% of eligible markets in 2010 to just 28% in 2022. Is that progress? Or are companies just getting smarter? Instead of launching their own generics, they’re now using legal settlements to buy silence. The method changed. The outcome didn’t.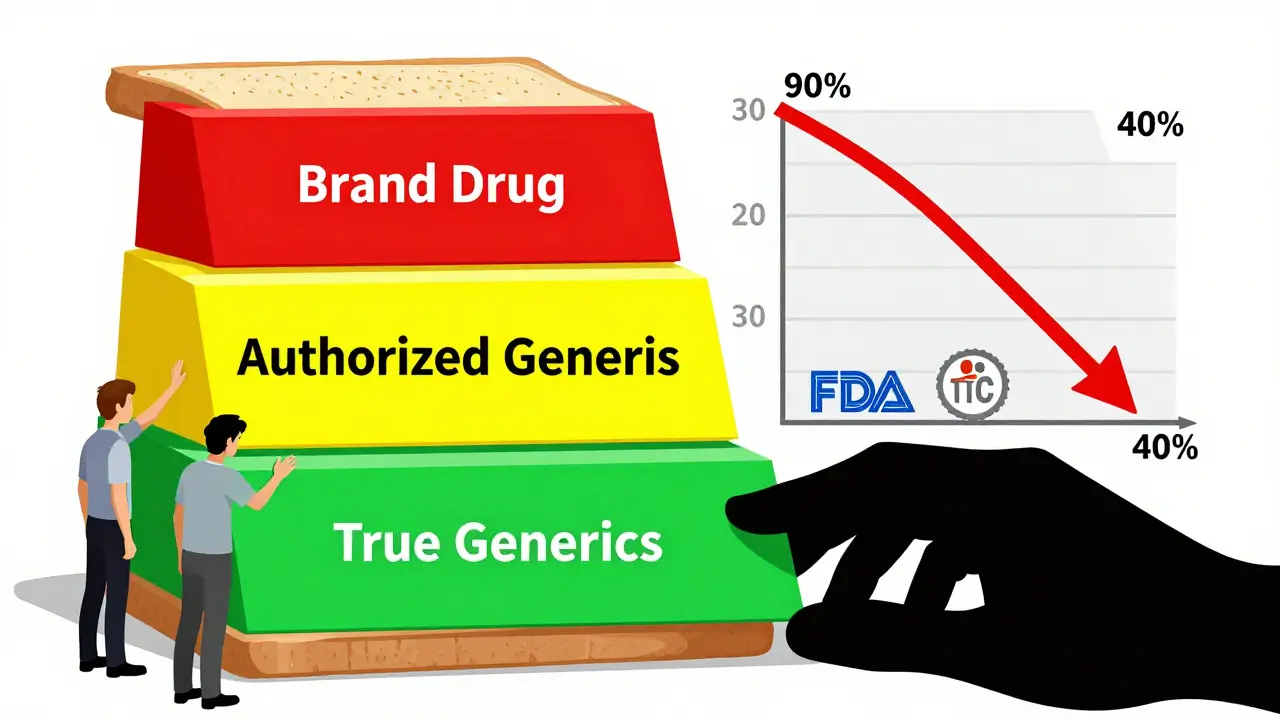
The Real Cost: Fewer Generics, Higher Prices
The Hatch-Waxman Act was supposed to be a win for patients. It gave us a system where competition drives prices down. But authorized generics turned it into a game where the brand company plays both sides. They win the patent lawsuit, then undercut the winner with their own version. For drugs with annual sales under $27 million, the threat of an authorized generic is enough to scare off generic challengers entirely. That’s 13% of all drugs-1% of total spending-but it’s still thousands of prescriptions that never get cheaper. And it’s not just about money. It’s about access. When generic companies can’t make a profit on challenging patents, they stop trying. That means fewer alternatives for patients. More reliance on brand drugs. Higher costs for everyone.What’s Next?
The trend is shifting. Authorized generics aren’t as common as they were. But the real threat now is the quiet agreements-settlements that delay entry without ever launching a generic. These are harder to prove, harder to stop, and even more damaging. The FTC is pushing harder. Congress is still debating. Generic manufacturers are still losing billions. And patients? They’re still paying more than they should. Until Congress closes the loophole-or the courts shut it down-authorized generics will remain a legal way for brand-name companies to protect their profits at the expense of competition.Are authorized generics the same as regular generics?
Yes, chemically and physically. Authorized generics are made by the brand-name company in the same facility using the same ingredients and process. The only difference is the label and packaging. Regular generics are made by independent companies that reverse-engineer the drug and file their own FDA applications.
Why do brand companies launch authorized generics?
To capture market share during the 180-day exclusivity period granted to the first generic challenger. By launching their own version, they prevent the independent generic from dominating sales. It’s a way to maintain revenue and pressure the generic company into accepting lower profits-or staying out of the market entirely.
Do authorized generics lower drug prices?
Not as much as real generics. Authorized generics are priced between the brand-name drug and true generics-usually 15-20% cheaper than the brand but 25-30% more expensive than independent generics. They fragment the market and prevent true price competition, which is what drives long-term savings for patients and insurers.
Is it legal for a brand company to launch an authorized generic during a generic’s exclusivity period?
Yes. The FDA and courts have ruled that the Hatch-Waxman Act doesn’t prohibit it. But while legal, it’s controversial. The FTC argues it undermines the law’s intent to reward patent challengers. Congress has repeatedly tried to ban the practice, but no law has passed yet.
How do authorized generics affect patent litigation?
They reduce the incentive to challenge patents. Generic companies spend millions to win the right to be the first to market. If they know the brand will launch its own generic right after, the potential return shrinks. Many now avoid filing challenges altogether, especially for lower-revenue drugs. This reduces competition and keeps prices high.
What’s being done to stop authorized generics?
The FTC is investigating agreements that delay authorized generic entry as anticompetitive. Legislation like the Preserve Access to Affordable Generics and Biosimilars Act has been reintroduced to ban such practices. Some states are also exploring legal actions. But without federal law, the practice continues, though it’s declining due to regulatory pressure and public scrutiny.






14 Comments