When a patient has been stable on a brand-name NTI drug for months or even years, switching to a generic version can feel risky-even if the science says it’s safe. NTI drugs are not like regular medications. A tiny change in blood levels-too little or too much-can mean the difference between control and crisis. For drugs like warfarin, phenytoin, or levothyroxine, even a 10% shift in absorption can trigger a seizure, a blood clot, or a thyroid crash. So when a pharmacist hands over a new bottle with a different label, the patient’s mind races: Is this really the same? Will I get sick?
Why NTI Drugs Are Different
NTI stands for narrow therapeutic index. That means the gap between the dose that works and the dose that harms is razor-thin. Take warfarin: the goal is to keep the INR between 2 and 3. Go below 2, and you risk a stroke. Above 3, and you could bleed internally. Digoxin? Therapeutic range is 0.5 to 0.9 ng/mL. One pill with slightly different absorption, and you’re in toxic territory. These aren’t hypotheticals. Between 2019 and 2023, over 1,200 adverse event reports were linked to NTI drug switches, mostly involving antiepileptics and anticoagulants.
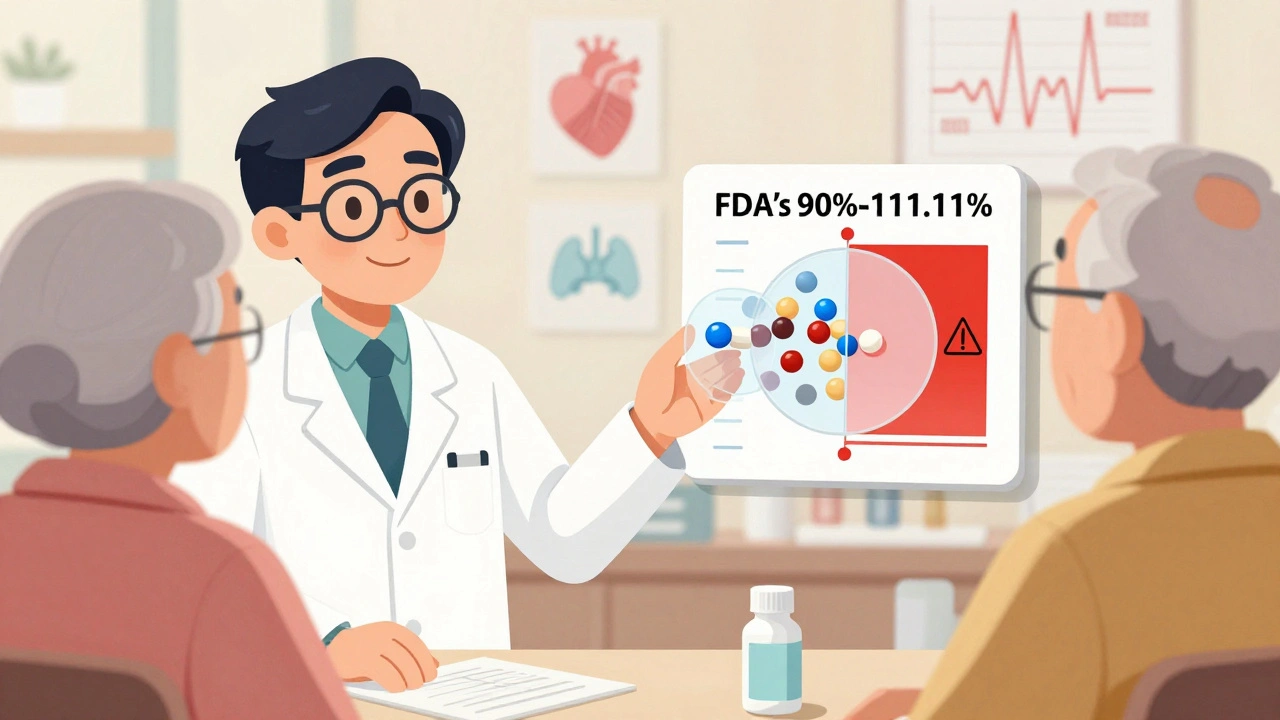
The FDA knows this. That’s why their standards for NTI generics are stricter than for regular drugs. While most generics must match brand drugs within 80%-125% of absorption, NTI generics must hit 90%-111.11%. For some, like levothyroxine, the bar is even higher: 95%-105% for total exposure. These aren’t arbitrary numbers. They’re based on real patient data, statistical models, and years of research from the FDA’s Narrow Therapeutic Index Working Group.
But Patients Still Don’t Trust Them
Here’s the problem: trust doesn’t come from data sheets. It comes from conversations. A 2017 national survey found that 94% of pharmacists believed NTI generics were safe-but only 60% routinely substituted them. Why? Because they’d seen patients panic after a switch. Some had seizures. Others had unstable INRs. Even if the lab results later showed no difference, the fear stuck.
And it’s not just pharmacists. Doctors hesitate too. One study showed that pharmacists with over 20 years of experience were 37% less likely to switch NTI drugs than newer practitioners. Why? Because they remember the cases where things went wrong-even if those cases were rare or unrelated to the generic itself. The human brain remembers fear more than statistics.
What to Say-And What Not to Say
Don’t say: “It’s the same thing.” That’s not true. It’s not the same pill. Different fillers, different coatings, different manufacturing. But it’s the same active ingredient, in the same amount, absorbed the same way-within FDA’s tight limits.
Instead, say: “This generic version has been tested to deliver the same amount of medicine into your bloodstream as the brand version. For your specific medication, we’ll check your blood levels in one week to make sure everything is working correctly.”
Use the “trusted advisor” approach, not the “librarian” approach. Don’t just hand out facts. Share your judgment. “I prescribe this generic for my own family. I’ve seen it work just as well-when we monitor it properly.”
And never assume the patient understands. Use the teach-back method: “Can you tell me why we’re checking your INR next week?” If they can’t explain it, you haven’t communicated clearly enough.
When Monitoring Is Non-Negotiable
Switching NTI drugs isn’t a one-time event. It’s the start of a short monitoring window. Here’s what the guidelines say:
- Warfarin: Check INR within 3-5 days after the switch. Some experts recommend a second check at 7-10 days.
- Phenytoin, carbamazepine: Serum level check within 7-10 days. Watch for new tremors, dizziness, or mood changes.
- Levothyroxine: Check TSH in 6-8 weeks. Don’t wait longer. Even small changes in absorption can throw off thyroid control.
- Digoxin: Check serum level and kidney function within 5-7 days. Older adults are especially sensitive.
Don’t just tell the patient to “watch for symptoms.” Tell them exactly what to watch for-and when to call. “If you feel your heart racing, dizzy, or confused, call us right away. Don’t wait for your next appointment.”
State Laws Can Change Everything
Even if you’re ready to switch, the law might not be. As of 2024, 27 U.S. states have special rules for NTI drug substitutions. Fourteen of them require written patient consent before a pharmacist can swap the brand for a generic. Thirteen others restrict substitution entirely for certain drugs.
That means your role isn’t just to educate-it’s to verify. Before you write a prescription for a generic NTI drug, ask: “Does my state allow automatic substitution for this drug?” If not, you need to specify “Dispense as written” on the script. Skip this step, and you risk a legal issue-even if the switch is clinically sound.
Who Needs Extra Care?
Not all patients are the same. Some are at higher risk when switching:
- Patients over 65: Slower metabolism, multiple medications, kidney changes.
- Those with liver or kidney disease: Alters how the drug is cleared from the body.
- People on multiple interacting drugs: Like a patient on warfarin who just started an antibiotic. That’s a recipe for an INR spike.
- Patients with a history of poor adherence: If they forget pills now, they won’t notice subtle changes in effect later.
For these groups, the monitoring window should be tighter. Consider checking labs earlier, scheduling a follow-up call within 48 hours, or even having the pharmacist do a home visit if possible.
Why Visual Aids Work
A 2023 survey found that pharmacists who used visual aids-like a simple chart showing the therapeutic range of warfarin, or a diagram of how the drug is absorbed-had 42% higher patient adherence than those who just talked.
Why? Because numbers on a page mean nothing. A line on a graph showing “safe zone” vs. “danger zone”? That sticks. Use the FDA’s free patient handouts. Print them. Point to them. Let the patient take one home. Say: “This is what we’re watching for. Keep it with your pill organizer.”
What Happens If You Don’t Talk About It?
Patients don’t ask questions because they assume the switch is safe-or because they’re afraid to seem difficult. But when things go wrong, they blame the doctor. “I switched to the generic, and now I’m having seizures.” “My INR went off the charts after I got the new bottle.”
Those aren’t just bad outcomes. They’re broken trust. And once trust is broken, it’s hard to rebuild. Patients may refuse future generics-even for non-NTI drugs. They may switch doctors. They may stop taking their meds altogether.
Effective communication isn’t optional. It’s the difference between a smooth transition and a medical emergency.
Final Checklist for Every NTI Switch
Before you prescribe or dispense a generic NTI drug, run through this:
- Confirm the drug is on the FDA’s NTI list (e.g., warfarin, levothyroxine, phenytoin).
- Check your state’s substitution laws-do you need written consent?
- Explain that the generic is not identical but is proven to work the same way within strict limits.
- Specify exactly which lab test to monitor and when (e.g., “INR in 4 days”).
- Describe warning signs: “If you feel X, call us immediately.”
- Use a visual aid or printed material.
- Use teach-back: “Can you tell me what you’ll do after you start this new pill?”
- Document the conversation: “Patient counseled on therapeutic equivalence of generic [drug], advised to monitor [test] within [timeframe].”
It takes 10 minutes. But those 10 minutes can prevent a hospital stay, a lawsuit, or worse.
What’s Next for NTI Drugs?
The FDA is launching a real-world monitoring system in 2025, using data from 12 million patients to track outcomes after brand-to-generic switches. Early results from pilot studies show that with proper monitoring, adverse events drop by over 60%. That’s huge.
But technology won’t fix this alone. The real solution is better communication-clear, consistent, and compassionate. Because when it comes to NTI drugs, the most powerful tool isn’t the lab machine. It’s the conversation between provider and patient.
Are generic NTI drugs really as safe as brand-name ones?
Yes-when they meet FDA’s stricter standards. For NTI drugs, generics must match the brand within 90%-111.11% of absorption, compared to 80%-125% for regular drugs. Some, like levothyroxine, must stay within 95%-105%. These aren’t guesses. They’re based on real patient data and statistical models. The FDA has approved over 37 drugs as NTI with these enhanced standards. But safety also depends on monitoring. A generic that’s bioequivalent won’t work if you don’t check blood levels after switching.
Why do some patients have problems after switching to a generic NTI drug?
Most of the time, it’s not the generic itself-it’s the lack of monitoring. Patients switch and assume everything’s fine. But with NTI drugs, small changes in absorption can build up over days. A patient on warfarin might not feel different until their INR hits 5.0, then they have a bleed. Or someone on phenytoin might develop tremors after a week because their blood level dropped slightly. The drug is fine. The system failed to catch the change. That’s why follow-up testing is mandatory, not optional.
Can I switch back to the brand if I’m worried?
Yes, but only if your doctor writes a new prescription with “Dispense as Written” or “Brand Necessary.” Insurance may not cover it unless you prove the generic caused a problem. Most insurers require you to try the generic first. If you switch back and forth between brand and generic, your blood levels will fluctuate. That’s dangerous with NTI drugs. Stick with one version unless your provider advises otherwise.
Do all pharmacies stock generic NTI drugs?
Most do-but not all. Some pharmacies avoid stocking them because of legal risks or fear of complaints. In states with strict NTI substitution laws, pharmacists may only dispense the brand unless they get written consent. If your pharmacy doesn’t have the generic, ask if they can order it. Or ask your doctor to prescribe a brand that’s covered under your plan. Don’t let pharmacy inventory decide your treatment.
What if my insurance forces me to switch?
Insurance companies often push generics to save money. But with NTI drugs, you have rights. If your doctor says the brand is medically necessary, they can file a prior authorization appeal. You can also ask for a one-time exception. Don’t accept the switch without a plan for monitoring. If you’re forced to switch and no one checks your labs afterward, that’s a failure of care-not a cost-saving win.
How do I know if my generic NTI drug is FDA-approved?
Look for the “AB” rating on the FDA’s Orange Book. For NTI drugs, it will say “AB1” or “AB2,” meaning it’s therapeutically equivalent. If it’s listed as “BX,” it’s not approved for substitution. You can check the FDA’s website or ask your pharmacist to show you the rating. Never take a generic without an AB rating for an NTI drug.
Is it okay to switch between different generic brands?
It’s not recommended. Even though each generic meets FDA standards, different manufacturers use different fillers and coatings. Switching between generics can cause small shifts in absorption-enough to matter with NTI drugs. Stay with one generic brand once you’ve stabilized. If you must switch, treat it like the first switch: monitor labs, watch for symptoms, and document everything.
What to Do Next
If you’re a provider: Print the FDA’s NTI counseling checklist. Put it on your desk. Use it every time. If you’re a patient: Ask your pharmacist for the written materials. Keep them with your pill box. If you’re a caregiver: Set phone reminders for lab appointments. Don’t rely on memory.
NTI drugs don’t need more science. They need better conversations. The data is clear. The standards are tight. What’s missing is the human step-the moment someone takes the time to say, “This matters. I’m watching with you.” That’s what keeps people safe.




14 Comments