Glaucoma doesn’t hurt. It doesn’t blur your vision suddenly. You won’t wake up one morning unable to see. That’s why it’s called the silent thief of sight. By the time most people notice something’s wrong, the damage is already done - and it’s permanent.
At its core, glaucoma is about pressure. Not the kind you feel in your head, but inside your eye. That pressure, called intraocular pressure (IOP), normally stays between 10 and 21 mmHg. When it climbs higher, it starts crushing the delicate fibers of your optic nerve - the cable that sends every image from your eye to your brain. Think of it like a garden hose under too much water pressure. Eventually, the walls crack. In your eye, those cracks are dead nerve cells. And once they’re gone, they don’t come back.
It’s Not Just About High Pressure
For decades, doctors thought glaucoma was just about high eye pressure. If your IOP was above 21 mmHg, you were at risk. If it was below, you were safe. That’s not true anymore.
Up to 30% of people with glaucoma have normal eye pressure. This is called normal-tension glaucoma (NTG). Their optic nerves still get damaged - even when their pressure readings are perfectly fine. Why? Because pressure isn’t the whole story. What matters more is the difference between the pressure inside your eye and the pressure in your brain. That’s called the translaminar pressure gradient. If your eye pressure is 18 mmHg but your brain pressure is only 10 mmHg, that’s a 8 mmHg push from behind your optic nerve. That’s enough to cause damage over time. In people with ocular hypertension (high pressure but no damage), brain pressure is often higher, balancing things out. That’s why some people with pressure at 28 mmHg never lose vision - and others with pressure at 14 mmHg do.
What Actually Gets Damaged?
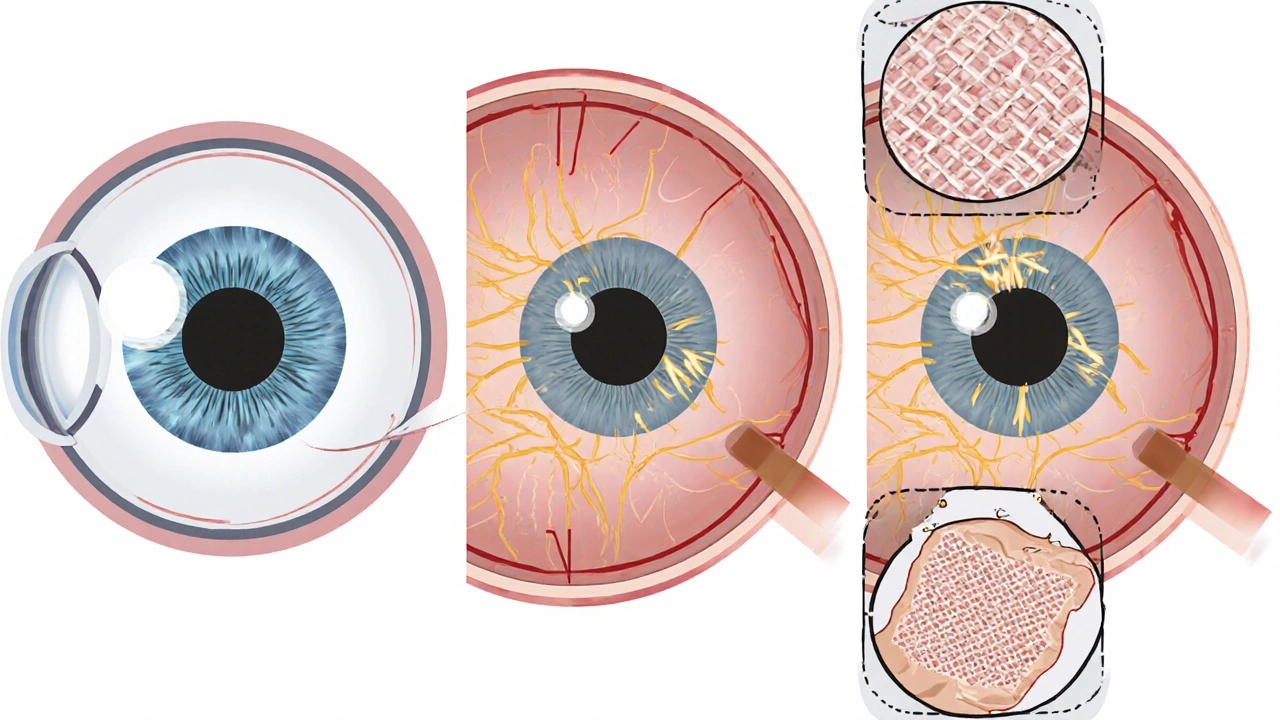
The real target isn’t your whole optic nerve. It’s a tiny structure at the back of your eye called the lamina cribrosa. It’s a sieve-like mesh made of connective tissue, and it’s where the nerve fibers exit your eye. When pressure rises, this mesh gets stretched. Studies using advanced imaging show that in glaucoma patients, the lamina cribrosa moves backward by 30-50% more than in healthy eyes. That stretching cuts off the blood supply to the nerve fibers and crushes them like a cable under a heavy door.
Those nerve fibers belong to retinal ganglion cells - the only cells in your eye that send visual signals to your brain. When they die, your visual field shrinks. First, you lose peripheral vision - the edges of what you see. You might bump into doorframes or miss the person standing beside you. Then, the damage creeps inward. By the time you notice your central vision is affected, you’re already at the late stage. That’s why early detection isn’t just helpful - it’s your only defense.
How Do Doctors Know You Have It?
Glaucoma doesn’t show up on a regular eye chart. You need three specific tests:
- Eye pressure measurement - using a device called a Goldmann tonometer. It gently touches your cornea after numbing drops. Normal is 10-21 mmHg, but your target depends on your baseline and how advanced your disease is.
- Optical coherence tomography (OCT) - this non-invasive scan takes a 3D image of your optic nerve and retina. It can detect thinning of the nerve fiber layer as small as 5-10 microns - thinner than a human hair.
- Visual field test - you stare at a center light while flashing dots appear around you. You press a button when you see them. This maps your blind spots. A loss of 1 dB (decibel) in sensitivity can mean early damage.
Many people hate the visual field test. It takes 15-20 minutes per eye. It’s boring. It’s tiring. But it’s the only way to track if your vision is getting worse. One patient on Reddit said, “I’ve had IOP at 12-13 for five years, and my field is still shrinking. That’s why I keep coming back.”
Types of Glaucoma - Not All Are the Same
There are two main types, and they need different approaches:
- Primary Open-Angle Glaucoma (POAG) - the most common form. It’s slow, silent, and affects 90% of cases in the U.S. The drainage angle of your eye looks normal, but fluid drains too slowly. Pressure builds over years.
- Normal-Tension Glaucoma (NTG) - happens even when pressure is normal. Often linked to low blood pressure, migraines, or autoimmune conditions. In Asia, it makes up over half of all glaucoma cases.
- Angle-Closure Glaucoma - less common globally but responsible for half of glaucoma blindness in Asia. It’s sudden. The iris blocks the drainage angle. Pressure spikes fast - you get eye pain, nausea, blurred vision. This is an emergency.
- Secondary Glaucoma - caused by other issues like steroid use, eye injuries, or inflammation. Pseudoexfoliative glaucoma, common in older adults, causes flaky material to clog drainage.
What’s scary is how easily people miss NTG. A 2022 survey of 1,200 patients found that 42% stopped their eye drops because they didn’t feel sick. “I’m not in pain,” they’d say. “Why keep using this?” But the damage keeps going - even at 12 mmHg.
Treatment: Lowering Pressure Works - But Not Always Enough
The best way to slow glaucoma? Lower your eye pressure. Studies show that reducing IOP by 30% cuts progression risk by 50%. The Early Manifest Glaucoma Trial proved it. The Collaborative Initial Glaucoma Treatment Study showed that getting pressure below 18 mmHg (instead of 22) made a huge difference.
First-line treatment? Eye drops. Prostaglandin analogs like latanoprost are the most effective - they lower pressure by 25-33% with just one drop a night. But they have side effects: darker eyelashes, sunken eyes, redness. One patient said, “I look like I’m always crying. My husband thinks I’m sad. I’m not. I’m just on my drops.”
If drops aren’t enough, lasers help. Selective laser trabeculoplasty (SLT) opens up drainage channels. It works for 75% of people, but the effect fades by 10% each year. Surgery is next. Trabeculectomy creates a new drainage path. It’s 85% successful in the first year. But it’s invasive. MIGS (minimally invasive glaucoma surgery) like iStent is less risky - it lowers pressure by 20-25% and can be done at the same time as cataract surgery.
And here’s the kicker: only 25% of people are still using their drops after two years. That’s not laziness. It’s forgetfulness. It’s cost. It’s side effects. It’s thinking, “I feel fine.” But glaucoma doesn’t care how you feel.
What’s New? Beyond Pressure
Doctors now know that lowering pressure isn’t always enough. That’s why research is turning to neuroprotection - protecting the nerve cells themselves.
The LIBERTI study in 2024 found that brimonidine eye drops slowed NTG progression 30% more than timolol - even when pressure was the same. That suggests brimonidine might protect nerves directly. Other trials are testing CNTF implants, which help nerve cells survive longer. In animal studies, a protein called oncomodulin regenerated 40% of damaged nerve fibers. That’s not in humans yet - but it’s the first time we’ve seen nerve repair in glaucoma.
Home monitoring is also coming. The Triggerfish contact lens can measure pressure 24 hours a day. AI is now analyzing OCT scans to catch early damage before your doctor even sees it. One 2023 JAMA study showed AI detected glaucoma with 94% accuracy.
What You Can Do
There’s no cure. But there’s control. Here’s what works:
- Get checked yearly after 40 - especially if you’re Black, Asian, or have a family history.
- Don’t skip your eye drops. Set phone alarms. Use pill organizers.
- Know your numbers. Ask for your IOP, OCT results, and visual field trends. Don’t just take “everything’s fine” as an answer.
- Manage your blood pressure. Low nighttime pressure can worsen NTG.
- Exercise. Moderate aerobic activity lowers IOP by 5-10 mmHg for hours.
- Don’t do head-down yoga poses. They raise eye pressure.
Glaucoma doesn’t have to mean blindness. But it demands attention. You can’t feel it. You can’t see it. But your doctor can. And with the right tools, you can keep your vision - for decades.
Can glaucoma be cured?
No, glaucoma cannot be cured. Once nerve cells die, they don’t regenerate. But with early detection and consistent treatment - usually lowering eye pressure - progression can be slowed or stopped in most cases. The goal isn’t to restore vision, but to preserve what’s left.
If my eye pressure is normal, can I still have glaucoma?
Yes. About 20-30% of glaucoma cases happen with normal eye pressure (10-21 mmHg). This is called normal-tension glaucoma. Damage occurs because of factors like low cerebrospinal fluid pressure, poor blood flow to the optic nerve, or genetic vulnerability. Pressure alone doesn’t tell the full story.
How often should I get tested for glaucoma?
If you’re over 40 and have no risk factors, get tested every 2-4 years. If you’re over 60, get tested every 1-2 years. If you’re Black, Asian, have a family history, or have diabetes or high blood pressure, start annual testing at 40. If you’ve been diagnosed, you’ll need checks every 3-6 months for pressure, yearly visual field tests, and OCT scans every 6-12 months.
Do glaucoma eye drops have serious side effects?
Yes, but they’re usually manageable. Prostaglandin drops (like latanoprost) can darken eyelashes, cause eye redness, or lead to sunken eyes. Beta-blockers (like timolol) can lower heart rate or worsen asthma. Some drops cause burning or stinging. If side effects are bad, talk to your doctor. There are many types of drops, and switching can help. Never stop without talking to your eye specialist - the risk of vision loss is far greater than the side effects.
Is surgery better than eye drops?
It depends. Drops are the first step because they’re non-invasive. But if your pressure stays too high despite drops, or you can’t use them consistently, surgery may be better. Trabeculectomy and MIGS (like iStent) can reduce pressure more than drops alone and reduce long-term medication use. Surgery isn’t a cure, but it can give you more control - especially if you struggle with daily drops.
Can lifestyle changes help with glaucoma?
Yes. Regular aerobic exercise - like brisk walking or cycling - lowers eye pressure by 5-10 mmHg for hours. Avoid holding your breath or doing head-down positions (like yoga inversions), which raise pressure. Don’t smoke - it reduces blood flow to the optic nerve. Eat leafy greens, which are rich in nitrates and linked to lower glaucoma risk. And manage conditions like high blood pressure and diabetes - they worsen nerve damage.


19 Comments